Early symptoms can be hard to define and may mimic the small lapses that many people experience, especially with age.
Early Symptoms
Alzheimer’s is a brain disease that usually affects thinking and memory in older people. Memory problems, especially remembering recent events, is often one of the first outward signs. Memory tends to get a little less sharp in most older folks, so in the earlier stages of the disease, it can be hard to tell if the symptoms a person experiences are related to normal aging or Alzheimer’s. However, over time, memory loss and other symptoms become much more severe.
“Something just wasn’t right with my mom”
In the film, we meet Wendy Cooper, a caregiver for her mother, who has Alzheimer’s Disease. Cooper describes her mother as a person who was previously functioning at a very high level, working as a dietician at a hospital in New York. Cooper said, “she was in charge, she was the supervisor.”
When her mother retired, Cooper noticed she struggled with things that used to be routine, like filling up the car with gas or driving around the neighborhood without getting lost.
Family members or others who are close to the affected person may start to notice very subtle changes, even as their loved one remains mostly unimpaired, including:
memory lapses
poor judgement
changes in mood
increased forgetfulness
increased difficulty with planning, reasoning and carrying out tasks
Small Changes
Companion Guide
A Silent Progression
We now know that harmful changes in the brain begin to occur years or even decades before noticeable symptoms appear in AD. At first, these changes are very subtle and nearly impossible to detect.
A region known as the hippocampus (highlighted in red on the brain images below) and an interconnected set of cells, called the hippocampal circuit are often the first to be affected. This brain region is vital to a person’s ability to store and bring back memories.
The hippocampus is comprised of two bilateral regions, one in each brain hemisphere (side). Here, the region is highlighted in red and shown from three viewpoints: Left: from the top of the head looking down; Center: From the side of the head (ear to ear); Right: From the back of the head looking forward. This type of brain image comes from a magnetic resonance imaging (MRI) scan.
What is a neuron?
A neuron is a type of cell in the brain. Neurons are heavily involved in many of the functions that allow us to survive and function. One of their most important abilities is transmitting signals from one cell to the next via action potentials. In this way, the brain is able to receive sensory input, such as sight or sound, from the outside world. Neurons are also involved in transmitting instructions to the rest of the body, for example, by coordinating movement. The storing and recall of memories, critical thinking, and decision making also rely on the healthy functioning of networks of neurons in the brain.
While neurons can take on many shapes, the most commonly known contains a cell body (center), an axon (the beaded “tail” pointing down in the picture) and dendrites, which look like tree branches. The cell body contains many important functions vital to keeping the neuron healthy, along with the cell’s genetic blueprints. It also produces neurotransmitters, chemicals that serve as messengers between neurons. Receptors on the surface of the dendrites receive information from other neurons. This information can then be passed on to other cells through the axon.
Each human has approximately 86 billion neurons. The brain is resilient to the loss of some of these neurons over the lifespan, however if too many are lost, a person may begin to experience difficulty maintaining healthy functioning.
Accumulating Damage
Two abnormal structures appear in the brains of people with AD: plaques and tangles. Both are dense, fibrous structures that become evident in people experiencing the symptoms of AD. The plaques are made up of amyloid beta peptides, strings of amino acids that are building blocks for proteins. The normal purpose of amyloid beta has not fully been characterized, but in a healthily functioning brain, the level of amyloid beta remains under control through processes that degrade and transport the peptides out of the brain. In AD, these processes are not effective at controlling amyloid beta and the substance begins to build up. The plaques appear outside of neurons in extracellular space.
The presence of amyloid beta alone does not seem to be associated with AD. An abnormal, deformed type of tau protein forms the fibrous tangles that are another indicator of AD. The tau tangles interfere with healthy synaptic functioning in ways that can lead to the death of the neuron. Additionally, the presence of toxic tau tangles appears to increase the toxicity of the amyloid beta plaques, creating a negative feedback loop.
An Infectious Disease?
AD damages the neurons which are vital to a healthy functioning brain. AD is not contagious in the traditional sense — you don’t catch it from another person or from a virus. However, once misfolded tau protein is present, it can spread via synaptic connections from one neuron to the next. “The infectivity is caused by the protein itself, a protein that converts from its normal structure into a misfolded, toxic structure”, says UVA Alzheimer’s researcher Dr. George Bloom. In this way, the negative changes in cellular behavior spread from one location in the brain to the next.
The build up of toxic substances provokes an inflammatory response in the brain. This inflammation harms neuronal function by damaging synapses. Eventually, cells will die.
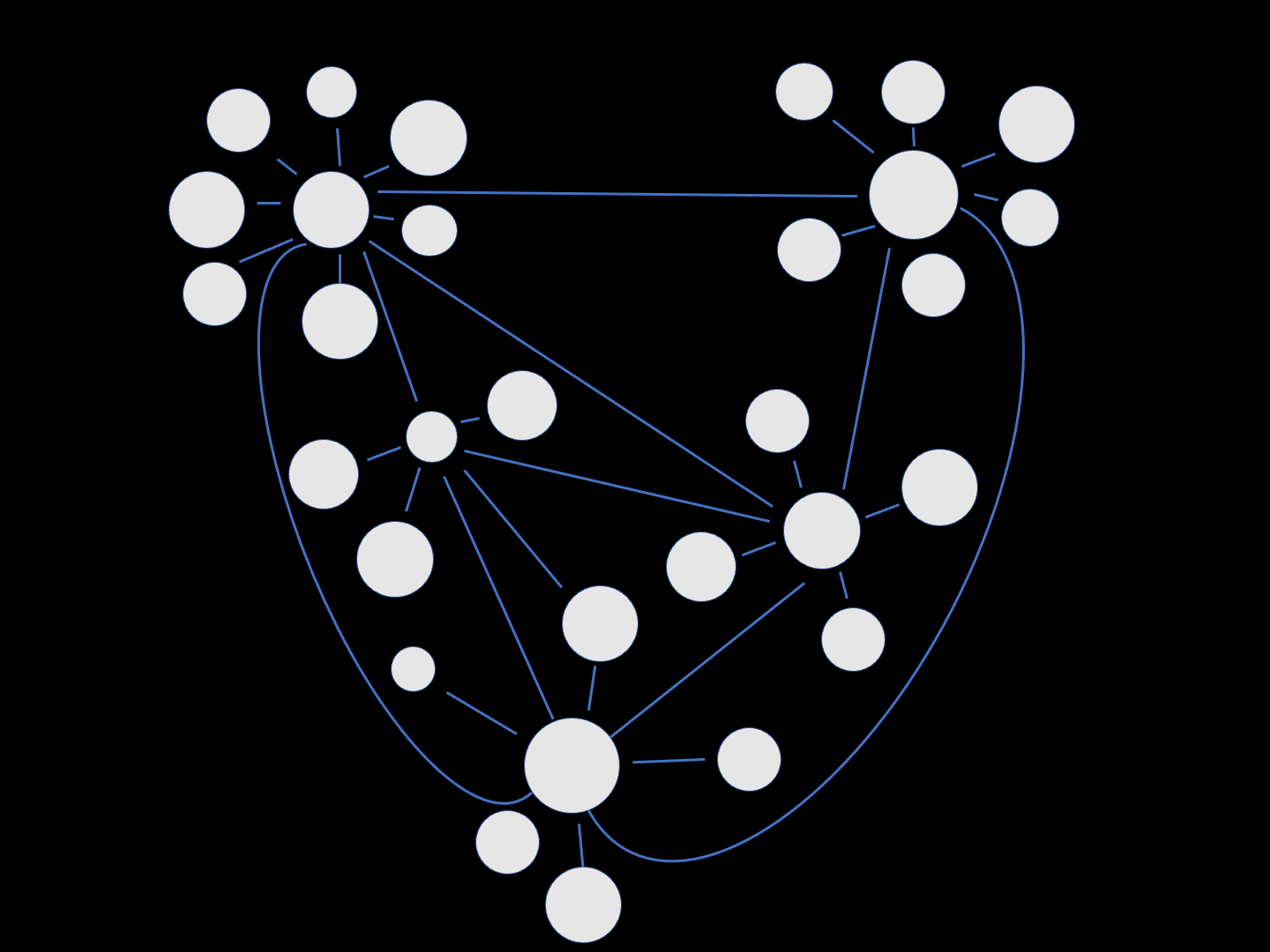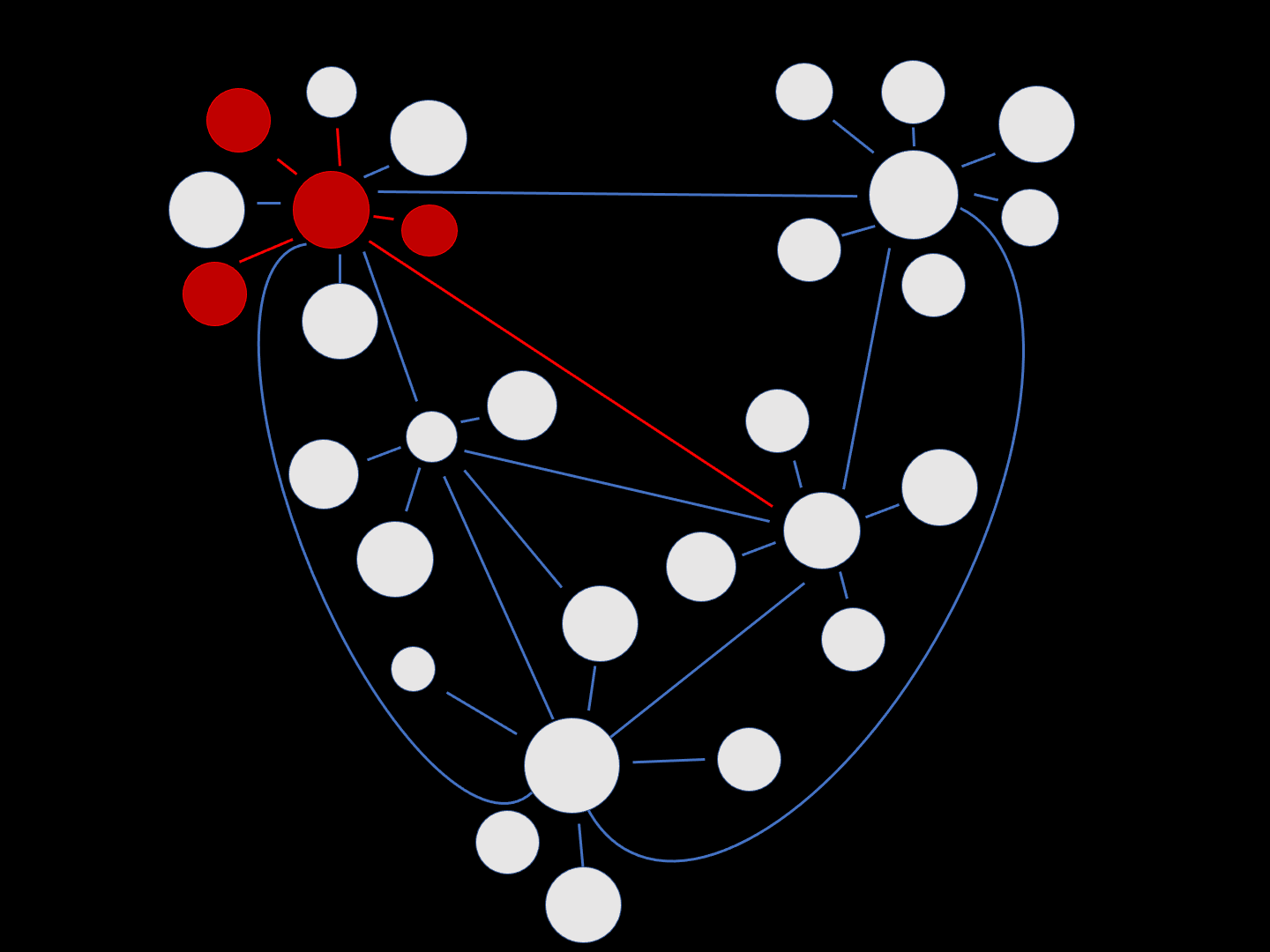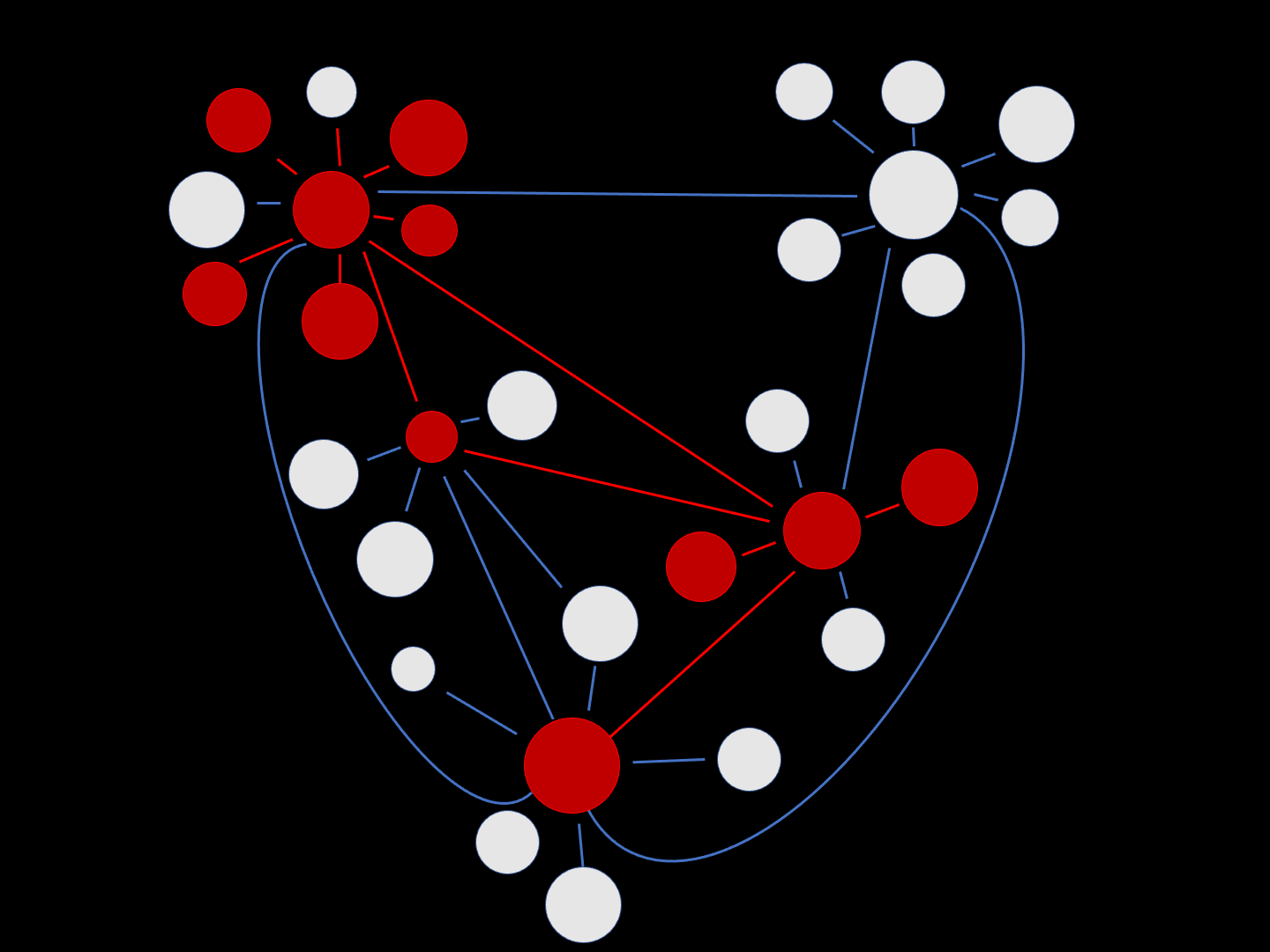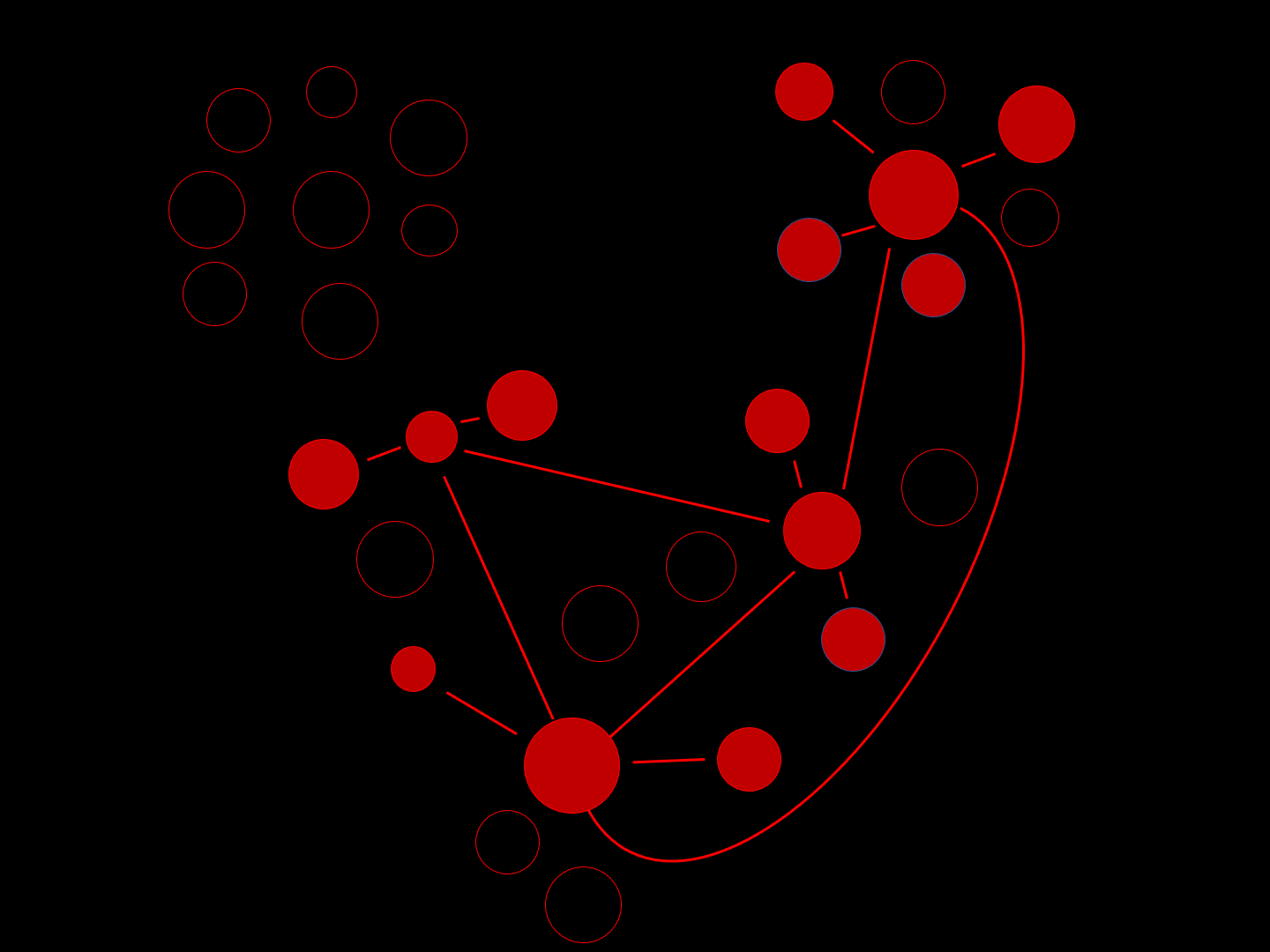
This schematic intuitively exemplifies how neuronal damage associated with AD can spread through a network of cells. The round white circles represent healthy neurons, with functional synapses (connections) depicted by the blue lines. Over time, neurons become damaged (red) and this spreads through the synaptic network.
“Misfolded protein spreads like wildfire in the brain.”
Research Directions
Risk Factors
Some Alzheimer Disease researchers focus on understanding individual risk factors. Identifying and responding appropriately to factors associated with Alzheimer disease may help prevent or delay the onset of disease in some people. Each person’s relative risk for developing the disease is determined by a mix of genetic, behavioral and environmental factors. Some of these risk factors are not under an individual’s control, others, while others may respond better to intervention. One challenge is in creating an environment where individuals are able to make healthy choices and receive appropriate medical advice and care, a process that involves coordinated efforts between scientists, medical and public health professionals, policy makers, patient advocates and individuals.
Support
Improving support people with Alzheimer Disease is a multi-faceted problem. Helping people in the earlier stages of AD to consider their goals and needs over the next stages of life can improve the patient’s sense of agency over their future. While treatment options for Alzheimer Disease are currently limited, care can include management of depression or anxiety and finding ways for the affected person to remain engaged. Support groups for people living with Alzheimer’s Disease can also help. Helping caregivers and reducing the burden of care are also potential avenues for research.
Earlier Identification
Identifying individuals most at risk for Alzheimer Disease may provide a longer window to intervene before the brain sustains more damage. This could help clinicians be more certain of their diagnoses at an earlier stage, helping patients better plan for the future and potentially enter clinical trials at an earlier stage. Preventing further damage may be easier than reversing damage, suggesting that diagnosis at the earliest stages of disease is likely to yield better outcomes.
Treatment
Despite decades of research, there are limited medical interventions to halt the progression of AD. Aducanumab is an FDA-approved medication. Its mechanism of action is to clear away amyloid beta plaques in the brain. Research was conducted in people with early stage AD or mild cognitive impairment (an even earlier precursor stage that may lead to AD), with some indication that cognitive decline slowed in those receiving the drug. However, use of the drug is controversial, with many arguing that the evidence for the drug’s effectiveness is very weak and that the benefit from using the drug is too minimal.
In September of 2022, promising results from a clinical trial of another drug, lecanemab, were announced. Similarly to aducanumab, the drug purportedly works by reducing the level of amyloid beta in the brain. In the clinical trial, patients with early stage AD had a slower cognitive decline than those who received a placebo.
Recent News
Alzheimer’s drug slows mental decline in trial – but is it a breakthrough?
Strong link found between normal-tension glaucoma, Alzheimer’s disease
With early Alzheimer’s in the family, these sisters decided to test for the gene
Aducanumab approved for treatment in Alzheimer’s Disease
Five Big New Questions About New Alzheimer’s Treatment
Symptoms and Stages
Mayo Clinic: Alzheimer’s Disease Overview
National Institute of Aging: Signs of Alzheimer’s Disease
Alzheimer’s Association: Stages
Support
Getting help with Alzheimers caregiving
Virginia Office of Aging Services
Scientific Reviews
Amyloid-B and tau: the trigger and bullet in Alzheimer disease pathogenesis (2014). Bloom.
Alzheimer’s Disease (2016). Scheltens et al.
Alzheimer Disease: An update on pathobiology and treatment strategies (2019). Long & Holtzman.
The Amyloid-β Pathway in Alzheimer’s Disease (2021). Hampel et al.